introductory
Did you know that Every year more than300,000 food facilitiesRegistration with the FDA is required, but as much as40% EnterprisesThere is a risk of having your products detained because you do not understand the registration requirements. For Hong Kong, Macao, and Taiwan enterprises that want to export food products to the U.S. market, it is important to knowFDA Food Registrationrespond in singingFDA Factory RegistrationThe complete process of FDA food facility registration is not only a necessary condition for compliance, but also a key to open up the U.S. market. In this article, we will provide you with a detailed analysis of the core concepts of FDA food facility registration, the application process, and frequently asked questions to help you successfully complete the registration process.
Summary of Core Points
- FDA Food RegistrationIt is a mandatory registration system required by the U.S. Bioterrorism Act.
- FDA Factory RegistrationNeeds to be updated every two years to ensure accuracy of information
- Facilities registered to cover all aspects of food production, processing, packaging and storage
- FDA has the authority to suspend or revoke a facility's registration under certain circumstances.
- Complete enrollment process including facility information submission, contact designation and e-verify

What is FDA Food Facility Registration?
Legal Background and Regulatory Basis
FDA Food RegistrationThe Public Health Security and Bioterrorism Prevention and Response Act (Bioterrorism Act) was passed by the U.S. Congress in 2002. According toU.S. FDA official regulationsThe Food Safety Modernization Act of 2011 (FSMA) further amended the registration requirements, authorizing the FDA to protect the U.S. food supply chain from the threat of terrorist attacks.Strengthened FDA's regulatory authorityThe
Registration Scope
FDA Factory RegistrationApplies to all food facilities that engage in the following activities:
- Manufacturing/Processing: alteration of the physical or chemical properties of food
- Packaging: Activity of putting food into containers
- Storage: Preservation of food for human or animal consumption
- distribution (of goods for sale): Facilities supplying food to the U.S. market
IMPORTANT: Farms, retail food establishments, restaurants, nonprofit food establishments, and meat and poultry product facilities regulated by the USDA are exempt from registration.
Core Requirements for FDA Food Facility Registration
Registration Period and Renewal Period
According toFDA Registration GuidanceThe provisions of the Ordinance:
| Registration Type | Time Requirement | Update Cycle |
|---|---|---|
| First Registration | Completed prior to facility operation | Not applicable |
| regular update | October 1 to December 31 of even-numbered years | Once every two years |
| Information Change | Within 60 days of the change | Live Updates |
List of mandatory information
fulfillmentFDA Food RegistrationThe following core information is required:
- Facility Information
- Facility name and full address
- Parent company information (if applicable)
- DUNS Number (Dun & Bradstreet Number)
- Contact Designation
- US Agent Information
- Emergency Contact Details
- Email and Phone Numbers
- Type of business activity
- Specific food processing activities
- Product Category Code
- Storage Type Description
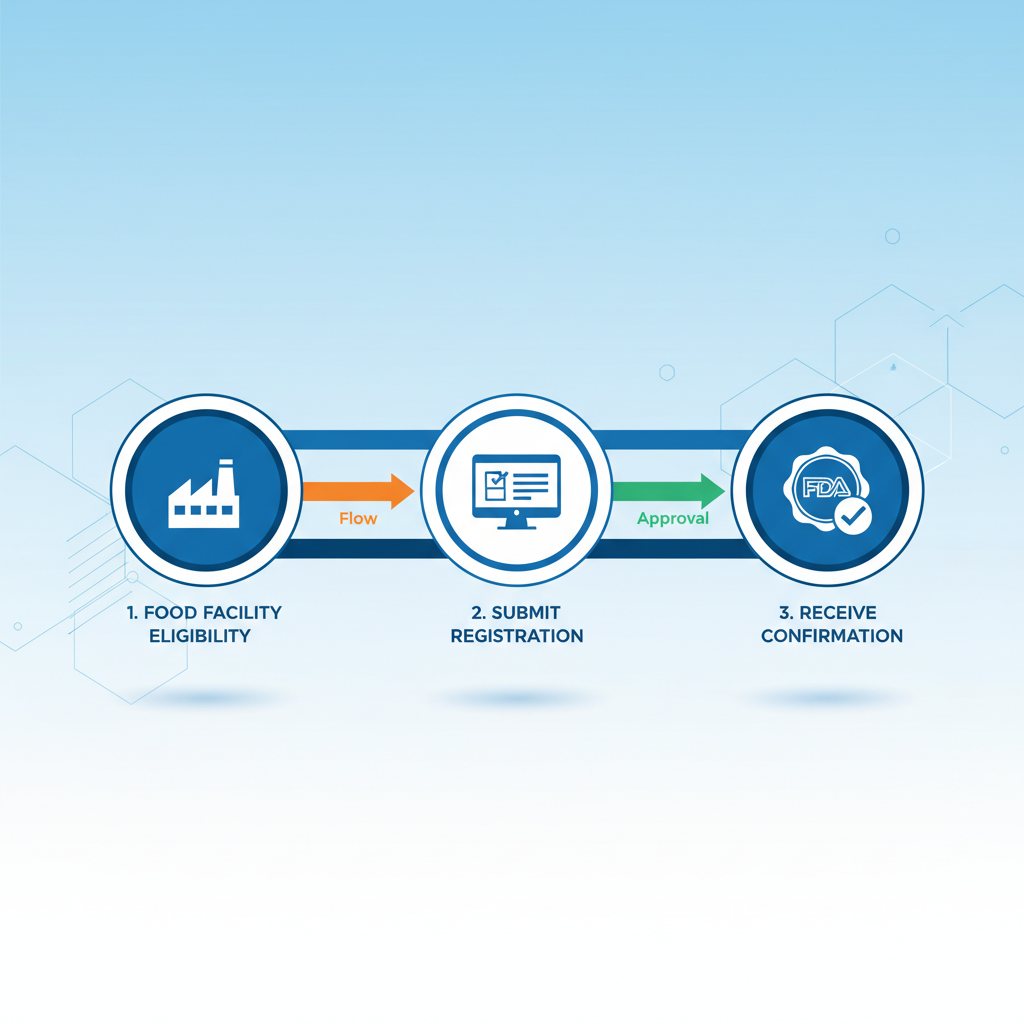
Application Process for FDA Factory Registration
Step 1: Prepare Registration Materials
At the beginningFDA Factory RegistrationBefore that, the business needs to be:
- Determine if the facility needs to be registered
- Prepare complete business and facility information
- Appointment of Qualified U.S. Agents
- Get DUNS number (available fromD&B Website(Application)
Step 2: Online Registration
FDA Food RegistrationAdoption of a fully electronic system:
- Access to the FDA Food Facility Registration System (FFR)
- Creation of FDA Industry System (FIS) Accounts
- Completing the Facility Registration Form (FDA Form 3537)
- Submitted and ReceivedRegistration Number(Registration Number)
Expert advice: The registration process usually takes 30-60 minutes and it is advisable to prepare all necessary documents in advance to increase efficiency.
Step 3: Follow-up Management Maintenance
According toFDA Compliance Guide::
- Updated every two years: Completed between October and December of even-numbered years
- Notification of Information Changes: Update facility address, contact person, etc. within 60 days of change.
- Deregistration: Facilities are required to proactively notify the FDA of discontinued operations.
Common Misconceptions and Points to Note about FDA Registration
Key Compliance Points
Many companies are conductingFDA Food RegistrationIt is easy to overlook the following points:
Registration is not the same as product certification
- FDA registration is a facility registration and does not mean that the product is approved.
- Certain products still require additional pre-market approval.
The Importance of U.S. Agents
- Must be a U.S.-based individual or business.
- Serve as a bridge between FDA and foreign facilities
- Can be an importer, distributor or third party service organization
Strict enforcement of renewal deadlines
- Failure to renew within the required time frame will result in the expiration of the registration.
- May be subject to product detention or refusal of entry.
FDA Suspension of Registration
Under FSMA authorization, FDA may suspend theFDA Factory Registration::
- Reasonable risk of health hazard or death from food
- Failure of the facility to provide the necessary inspection cooperation
- Serious violations of food safety laws and regulations
Conclusion
FDA Food Registrationrespond in singingFDA Factory RegistrationUnderstanding the complete registration process and compliance requirements can help Hong Kong, Macao and Taiwan enterprises avoid unnecessary trade barriers. Remember the three core points:Register in time,regular update,Accurate maintenance informationIt is recommended that companies establish an internal compliance management system or seek professional FDA registration consulting services to ensure consistent compliance with US food safety regulations. It is recommended that companies establish an internal compliance management system or seek professional FDA registration consulting services to ensure consistent compliance with U.S. food safety regulations.
Act Now: Review the status of your facility's registration to ensure that all necessary procedures are completed before the next renewal cycle to safeguard your eligibility to enter the U.S. market!
Frequently Asked Questions (FAQ)
Q1:How long does it take for FDA food registration?
fulfillmentFDA Food RegistrationTime depends on the level of preparation. The online registration system usually takes 30-60 minutes to operate, but it may take 1-2 weeks to prepare all the necessary documents (e.g., DUNS number, U.S. agent information). Registration is effective immediately after submission and a registration number will be issued immediately.
Q2:Do I need to pay for FDA factory registration?
No need.FDA Factory RegistrationIt is free in itself and the FDA does not charge any registration fees. However, if a company appoints a third-party consultant or agent to assist in the registration, it may need to pay a service fee. It is recommended to choose a reputable professional organization to ensure the accuracy of registration.
Q3: What are the consequences of forgetting to renew my FDA registration?
Failure to update within the required timeFDA Food RegistrationThis will result in an invalidation of the registration. Consequences include: products being detained at U.S. Customs, inability to enter the U.S. market, and possible fines. A new registration application will need to be filed to restore compliance, affecting normal trading activities.
Q4:Does a company with several factories need to register separately?
Yes. Each facility engaged in the manufacture, processing, packaging, or storage of food is required to be individuallyFDA Factory RegistrationThe facilities must have separate registration numbers for each physical address, even if they are owned by the same parent company. Even if these facilities belong to the same parent company, each physical address must have a separate registration number. However, the same U.S. agent may be designated.
Q5: How to verify the authenticity of FDA registration?
This can be done byFDA Official WebsiteTo verify the registration status of a facility, enter the registration number or facility name. Enter the registration number or facility name to check. It is recommended to check the registration status regularly to ensure that the information is accurate and within the validity period to avoid any registration problems affecting your export business.
Popular Articles:2026 Updated Guidance|How to Prepare for FDA Food Certification for Small and Medium-sized Enterprises? Process, Documents and Common Mistakes in One Go!